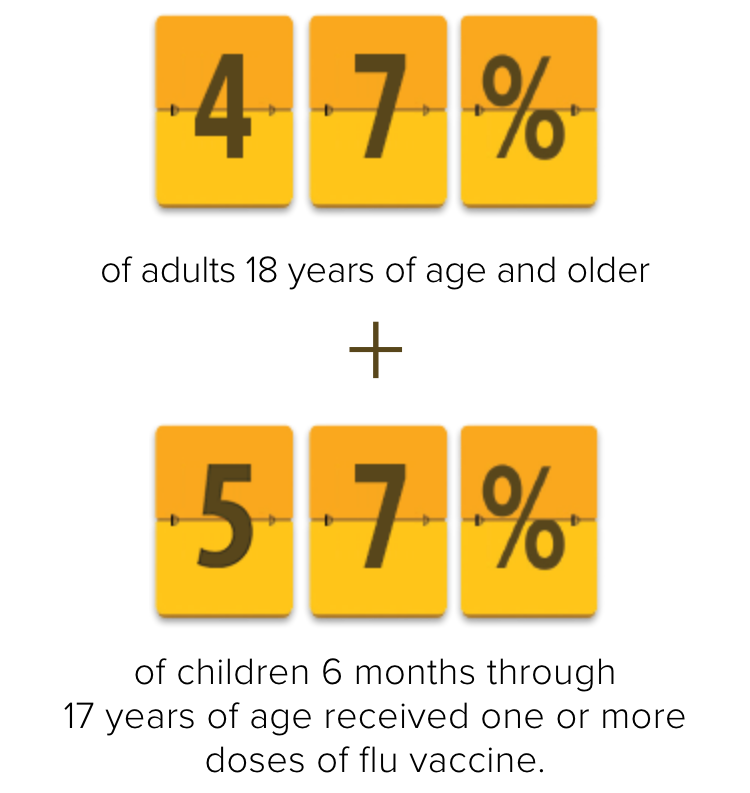
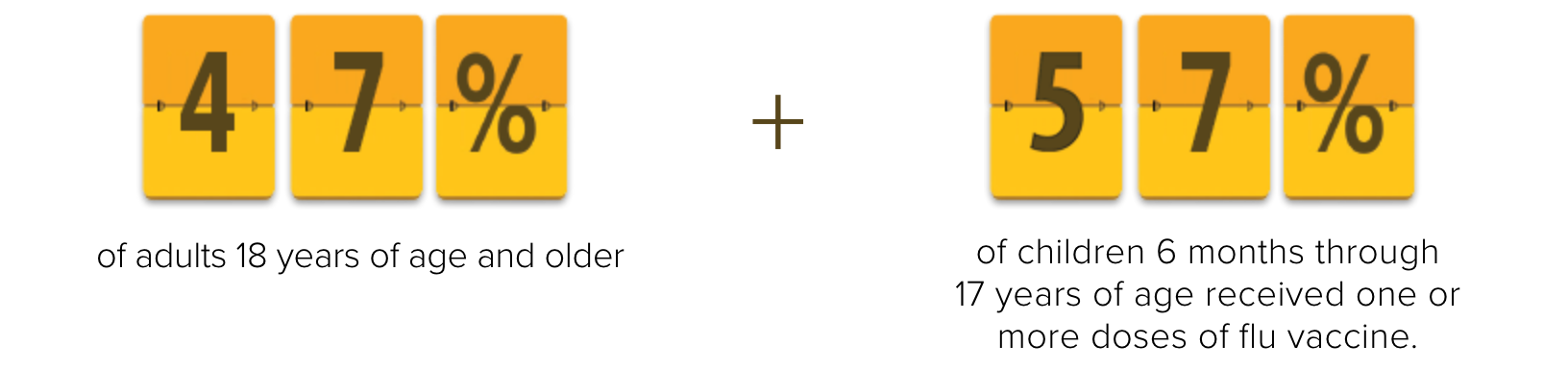Indication for FLUARIX and FLULAVAL
FLUARIX and FLULAVAL are vaccines indicated for active immunization for the prevention of disease caused by influenza A subtype viruses and type B virus contained in the vaccines. FLUARIX and FLULAVAL are approved for use in persons aged 6 months and older.
FLUARIX and FLULAVAL are vaccines indicated for active immunization for the prevention of disease caused by influenza A subtype viruses and type B virus contained in the vaccines. FLUARIX and FLULAVAL are approved for use in persons aged 6 months and older.
FLUARIX and FLULAVAL are vaccines indicated for active immunization for the prevention of disease caused by influenza A subtype viruses and type B virus contained in the vaccines. FLUARIX and FLULAVAL are approved for use in persons aged 6 months and older.
Important Safety Information for FLUARIX and FLULAVAL
- Do not administer FLUARIX or FLULAVAL to anyone
Do not administer FLUARIX or FLULAVAL to anyone with a history of severe allergic reactions (eg, anaphylaxis) to any component of the vaccine, including egg protein, or following a previous dose of any influenza vaccine
If Guillain-Barré syndrome has occurred within 6 weeks of
Do not administer FLUARIX or FLULAVAL to anyone with a history of severe allergic reactions (eg, anaphylaxis) to any component of the vaccine, including egg protein, or following a previous dose of any influenza vaccine
If Guillain-Barré syndrome has occurred within 6 weeks of receipt of a prior influenza vaccine, the decision to give FLUARIX or FLULAVAL should be based on careful consideration of the potential benefits and risks
Syncope (fainting) can occur in association with administration of injectable vaccines, including FLUARIX and FLULAVAL. Procedures should be in place to avoid injury from fainting
Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of FLUARIX and FLULAVAL
If FLUARIX or FLULAVAL is administered to immunosuppressed persons, including individuals receiving immunosuppressive therapy, the immune response may be lower than in immunocompetent persons
The most common (≥10%) solicited local adverse reactions with FLUARIX in adults were pain (55%) and redness (18%), and the most common systemic adverse reactions were muscle aches (23%), fatigue (20%), and headache (19%). In children aged 5 through 17 years, the most common (≥10%) solicited local adverse reactions were pain (56%), redness (18%), and swelling (14%), and the most common systemic adverse reactions were muscle aches (29%), fatigue (20%), and headache (15%). In children aged 3 through 4 years, the most common (≥10%) solicited local adverse reactions were pain (35%), redness (23%), and swelling (14%), and the most common systemic adverse reactions were irritability (21%), loss of appetite (13%), and drowsiness (13%). In children aged 6 through 35 months who received FLUARIX QUADRIVALENT, the most common (≥10%) solicited local adverse reactions were pain (17%) and redness (13%), and the most common systemic adverse reactions were irritability (16%), loss of appetite (14%), and drowsiness (13%)
The most common (≥10%) solicited local adverse reactions with FLULAVAL in adults were pain (51%), redness (13%), and swelling (11%), and the most common solicited systemic adverse reactions were fatigue (20%), headache (18%), and muscle aches/arthralgia (18%). In children aged 3 through 17 years, the most common (≥10%) solicited local adverse reaction was pain (56%). In children aged 3 through 4 years, the most common (≥10%) solicited systemic adverse reactions were irritability (25%), drowsiness (19%), and loss of appetite (16%). In children aged 5 through 17 years, the most common (≥10%) solicited systemic adverse reactions were muscle aches (24%), headache (17%), and fatigue (17%). In children aged 6 through 35 months who received FLULAVAL QUADRIVALENT, the most common (≥10%) solicited local adverse reaction was pain (40%), and the most common solicited systemic adverse reactions were irritability (49%), drowsiness (37%), and loss of appetite (29%)
Vaccination with FLUARIX or FLULAVAL may not result in protection of all vaccine recipients